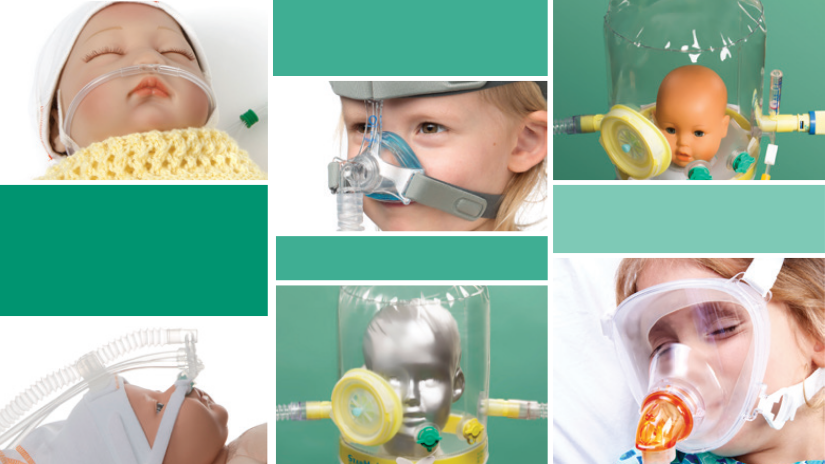
Importance of NIV in Paediatric and Infant Respiratory Diseases
Respiratory diseases are among the leading causes of hospitalisation and mortality in infants and children worldwide. Conditions such as bronchiolitis, pneumonia, asthma exacerbations, and neuromuscular disorders can quickly compromise breathing, making early and effective respiratory support essential.Nasal High Flow (NHF), Continuous Positive Airway Pressure (CPAP) and Non-invasive Ventilation (NIV) therapies can provide a safe and effective means of supporting breathing without the need for endotracheal intubation. By delivering positive airway pressure through a nasal interface, a mask or a helmet, NIV improves gas exchange, reduces the work of breathing, and helps prevent respiratory muscle fatigue. Its use is expanding across neonatal and paediatric intensive care units, emergency departments, and even home settings, reflecting its versatility and clinical value. daugiau

VT Select Bag-Valve-Mask (BVM) Resuscitators
The latest addition to our BVM offering. daugiau
Brand Values
We’re pleased to share news of our recently refreshed brand values, which were previously centred on choice. daugiau
Introducing the new improved Universal Stylet Bougie (USB™)
The Universal Stylet Bougie (USB™) - a hybrid stylet and bougie. daugiau
EcoVadis™ Approved
We are thrilled to announce that Intersurgical has been awarded the EcoVadis Committed Badge! daugiau
'7 steps' in anaesthesia
The '7 steps' to help improve patient and environmental safety in anaesthesia. daugiau
Access IFUs and Product Documentation online
Find out how to access our Product Documentation System to download supporting product documents. daugiau
DuoFlow™ bi-lumen breathing system range extension
Now available - DuoFlow™ bi-lumen breathing systems in 3m length configurations. daugiau


























